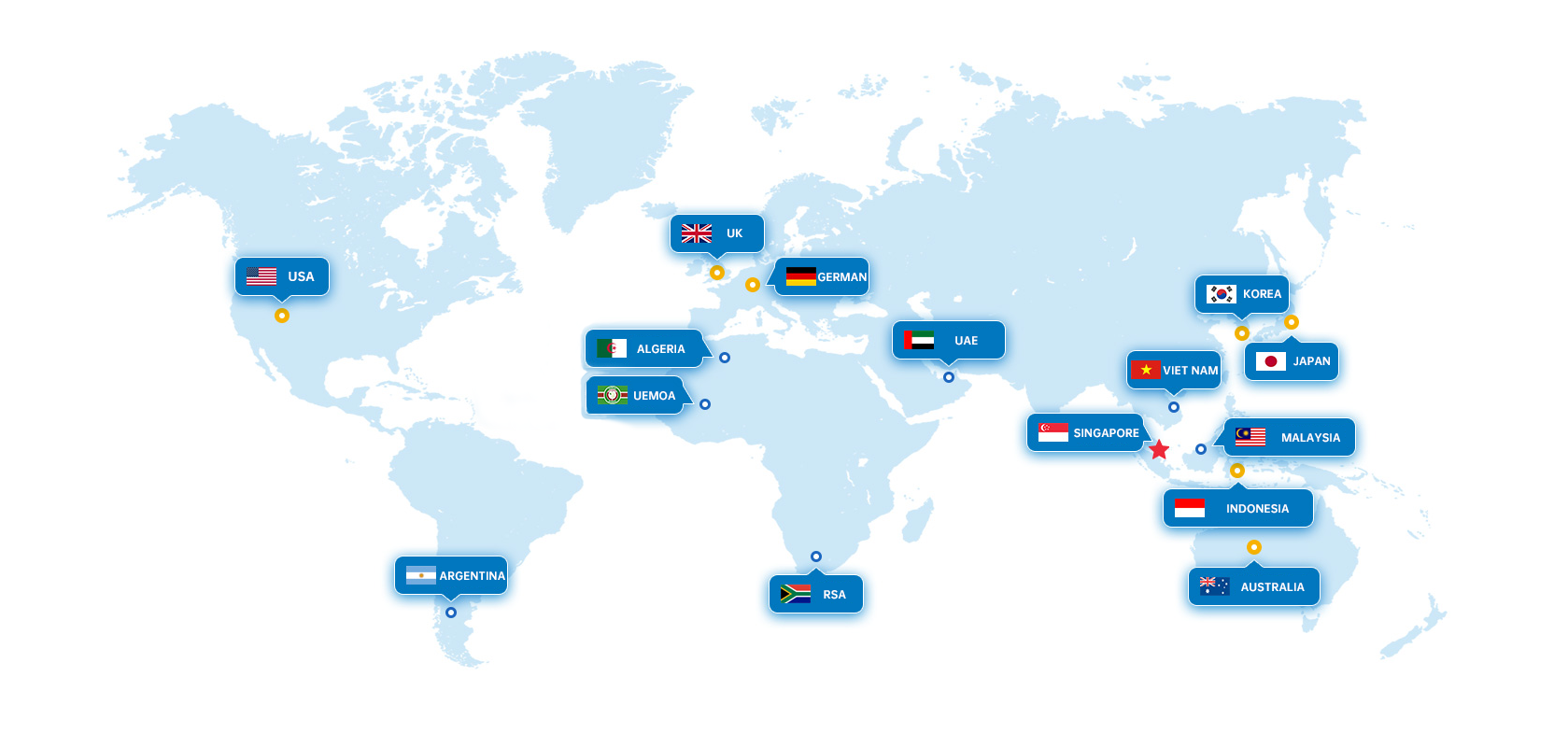

Pentagrams indicate global sales headquarters

Yellow circles indicate branch locations

Blue circles indicate sales partnership countrie


Four new drugs completed Phase I clinical trials in the United States and Australia, two of which have obtained FDA Orphan Drug Designation.
BLAX Project
FDA Orphan Drug Program
Overseas Clinical Program
First Generic in the US
International Drug Approvals
Insulin Glargine
Yinfenidone
Insulin Glargine
HEC88473
Yinfenidone
HEC96719
Linagliptin and Metformin Hydrochloride Tablets
Linagliptin Tablets
Ticagrelor Tablets
Fingolimod Capsules
Apixaban Tablets
TAF Tablets
Alogliptin Tablets
Brivaracetam Tablets
The company has two production bases located in Songshan Lake, Dongguan, Guangdong province, the PRC, and Yidu, Hubei province, the PRC, with a total area of over 1,300 mu. These production bases cover the entire production chain of formulations. Songshan Lake production base is an advanced factory in China producing solid chemical formulation and biologics. It has obtained GMP certifications from the United States, the European Union and China, including recently passing EU GMP audit conducted by National Office for Health and Social Affairs of Germany in November 2023, GMP inspection by the U.S. FDA in March 2024, and a GMP compliance check by the Guangdong Provincial Drug Administration in January 2025. Its annual production capacity of chemical drugs reaches 1.8 billion tablets/capsules. A large-scale biologics facility that complies with international GMP standards is expected be completed in 2026, equipped with production lines for cell, E coli fermentation and yeast fermentation as planned, which will provide solid support for the commercialization of our biologics under development. Yidu production base has obtained Chinese GMP certification, and it produces a wide range of insulin products, solid dosage forms and freeze-dried powder injections. As of December 31, 2024, Yidu production base was the largest production base of oseltamivir phosphate formulation in the PRC and can also produce a wide range of insulin products ranging from the second to fourth generation, with an annual production capacity of over 15 million injections. The company has already expanded its maximum annual insulin production capacity from 18 million injections in 2023 to 100 million injections in 2024. As of December 31, 2024, the annual theoretical production capacity of the Yidu chemical solid formulation production facility had passed 3.5 billion tablets/capsules, 1.6 billion granule packets and 4.5 million vials of freeze-dried powder injections.
The production bases adopt a comprehensive quality management system to ensure that the quality of our products meets the highest standards.